New policy lifts mandatory port testing for orphan drugs, accelerating treatment availability for rare disease patients.
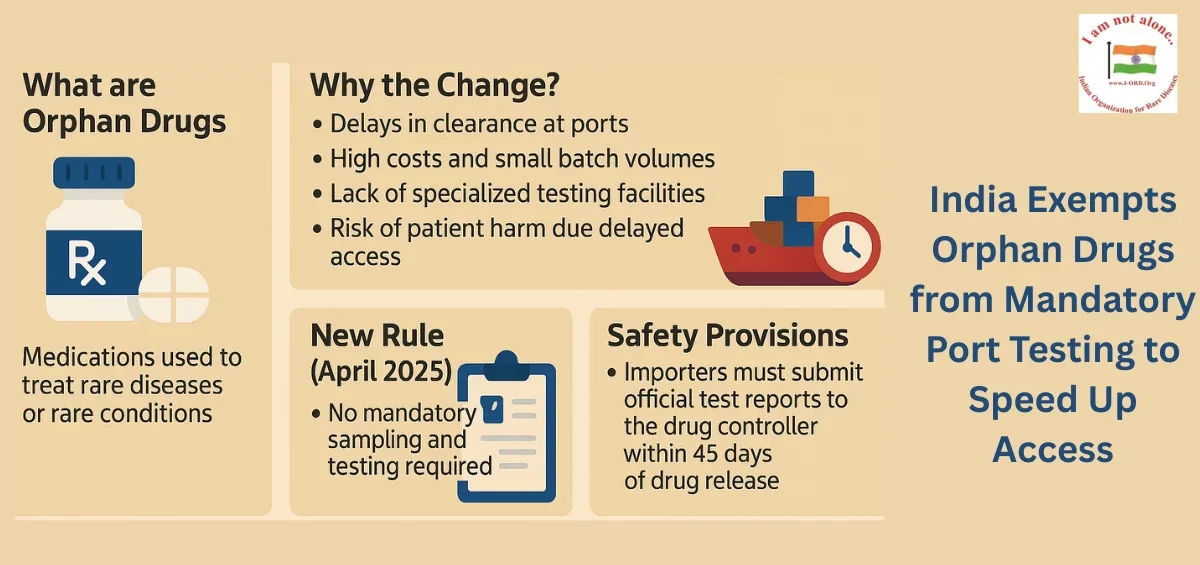
New Delhi: In a significant policy shift aimed at ensuring quicker access to life-saving treatments, the Government of India has exempted orphan drugs from mandatory sampling and testing at port offices. The Central Drugs Standard Control Organisation (CDSCO) announced on April 25, 2025 that consignments of orphan drugs will now be released based on a legal undertaking by importers, without waiting for testing results. This move is intended to mitigate delays in drug availability for patients suffering from rare diseases across India.
Under the new directive, importers must submit a legal undertaking along with forwarding the required sample quantity to designated laboratories. Importers are obligated to provide the test reports to the respective port offices within 15 days of receipt. The decision follows multiple representations from pharmaceutical associations and importers highlighting persistent logistical hurdles at ports, especially for small-volume, high-cost orphan drugs.
Orphan drugs, defined as treatments for conditions affecting fewer than five lakh people in India, often face challenges such as specialized equipment needs, limited reference standards, and complex testing procedures. These issues have previously caused critical delays, potentially endangering patients’ lives.
Rare disease advocacy groups and patients’ organisations have welcomed the government’s move. In a news report by The Economic Times, an executive from a children’s orphan disease association is quoted stating, “Several representations to the government were made on this issue and we are glad that a decision has been taken.”
The exemption is expected to dramatically reduce wait times for rare disease therapies, offering hope to thousands of patients who rely on timely treatment to manage debilitating and life-threatening conditions.
In its report, The Economic Times highlighted that small shipment sizes, high costs, and the unavailability of testing infrastructure had been causing undue hindrances to rare disease patients. The exemption aims to strike a balance between safeguarding public health and ensuring timely delivery of essential medicines.
Due to limited availability of orphan drugs, with only a small percentage of global orphan therapies being accessible domestically, the rare disease treatment landscape in India has been constrained. Hence, streamlining the import process is expected to improve outcomes for patients and reduce systemic bottlenecks.
Highlights:
- The Indian drug regulator CDSCO has exempted orphan drugs from mandatory testing at ports to speed up patient access.
- Importers must submit a legal undertaking and follow up with laboratory test results within 15 days.
- The move addresses longstanding challenges related to small consignment volumes, high costs, and specialized testing needs.